In this article, we will be giving you a complete overlook of the lewis structure of the H2O molecule. As we all know H2O is the molecular formula of Water, which is a major important constituent of earth. It also has a chemical name i.e. dihydrogen monoxide. An interesting thing is H2O is a single water molecule that is made of two hydrogen atoms and one oxygen atom. And the atoms are bonded by covalent bonds to form an H2O molecule.
Whereas, to form a compound, two or more H2O molecules join with a hydrogen bond. Due to the strength of the hydrogen bond, it has high melting and boiling point. However, we all know a covalent bond is stronger than a hydrogen bond, that’s why it readily reacts with most of the chemical elements of the periodic table.
H20 Lewis Structure
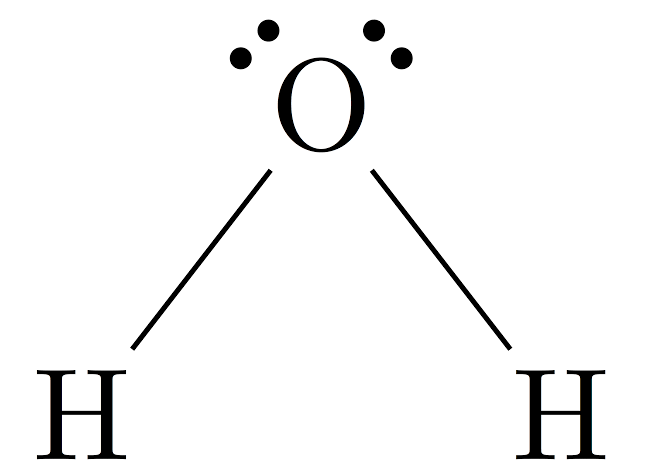
The lewis dot structure is also called electron dot structure, it gives a diagrammatic representation of the valence electrons present in each atom. And how they form bonds to form a molecule later the molecules bond together to form compounds. Similarly, the lewis dot structure of H2O will help us visualize the bonding and non-bonding between the electrons as well as the lone pair present in the molecule.
The lewis dot structure of H2O has a unique bent structure, it’s because it has two lone pairs on the central oxygen atom which increases electron repulsion. We will discuss this further below. So keep on reading it till last to point down all important points.
H2O Valence Electrons

Basically, the electrons which are present in an outermost shell of an atom and responsible for the chemical properties in the atom are called Valence Electrons.
- Atomic no. Of Hydrogen: 2
Electronic configuration of Hydrogen: 1s1
In the outermost shell of hydrogen, there is one electron. So the valence electron of hydrogen is 1. Since in H2O, two hydrogens are there so the total no. of valence electrons of Hydrogen is 2.
- Atomic no. Of Oxygen: 8
Electronic configuration of oxygen: 2s2 2p6
The central atom that is oxygen has 6 electrons in its outermost shell. So the valence electron of oxygen is 6.
Hence, in H2O, a total of 8 valence electrons are available to fill and complete the Octets of hydrogen and oxygen.
If you don’t know what the octet rule is, then let us explain you in short. The octet rule indicates that when the atoms have 8 electrons in their outermost shell they are most stable. The octet rule states that the atoms gain and lose electrons from their outermost shell to acquire the nearest noble gas configuration.
What is Lewis Structure Of H2O?
The structure of the Water molecule is quite simple than other complex molecules. Lewis structure of H2O is drawn in such a manner that it will satisfy the deficiency of each atom.
There are two single bonds around the central oxygen atom. Hydrogen molecules join with oxygen atoms with these single bonds to form a triatomic water molecule.
How to draw the lewis structure of H2O?
Follow the steps given below to draw the lewis dot structure of H2O easily.
- Look at the total no. of valence electrons present in the H20 molecule. Hydrogen has 2 valance electrons and Oxygen has 6. So the total no. of valence electrons present in a single water molecule is 8.
- Now decide which atom will be the central atom. Always remember the atom with a higher no. of valence electron will be the central atom. So, in H2O, oxygen will be the central atom.
- Now determine the total no. of electron pairs in H2O.
- Electron pair= total no. of valence electrons/ 2. In water, electron pair= 8/2= 4
- Now, let’s check the lone pairs.
Hydrogen has 1 electron in its outermost shell whereas oxygen has 6. In order to satisfy the octet rule, the oxygen atom needs 2 electrons and 2 hydrogen atoms need 1 extra each.
And how will they get that? So oxygen is going to share an electron with each of the hydrogens in exchange for hydrogen sharing its electron back. So they pair up to form two single bonds. And oxygen is left with 4 lone pair electrons and hydrogen with zero. They share a covalent bond in the molecule.
Hence, the required lewis structure is
Hybridization of H2O molecule:
In the Lewis structure of H2O, the oxygen and hydrogen atoms share a sigma bond with each other. And since sigma bonds are the strongest covalent bond the stability between oxygen and hydrogen is very high. The hinds leave two lone pairs on oxygen atoms which makes the difference.
The hybridization of an oxygen molecule is sp3. The oxygen atom in the H2O molecule has one s orbital and 3 p orbitals. These four orbitals combine and form 4 sp3 hybridized orbitals.
H2O Geometrical Structure:
The geometry of any molecule depends upon its lewis structure. The structure of the H2O molecule is bent (v-shaped). In H2O, the oxygen and hydrogen atoms are arranged symmetrically in a plane. However, the 4 lone pairs of electrons present on the oxygen push the atoms.
We all know the ideal bond angle for a bent molecule is 109.5°. But with an increase in lone pair the bond angle decreases. Since oxygen has 4 lone pair electrons over it, the angle reduces to 104.5°.
FAQ
1. How many bonds does H2O have?
There is two O-H single bond.
2. Does H2O follow the octet rule?
Yes H2O follows the octet rule.
3. What is the total number of lone pairs present in H2O?
Oxygen is the only atom in the H2O molecule which has 4lone pair electrons over it. Because hydrogen has already made a bond with the valence electrons of it.
4. Why is the molecular structure of H2O bent?
The shape of the water molecule is bent because of the lone pair present over the oxygen atom. These lone pair electrons push the atom forming a v-shaped bent structure.
5. Name other structures or molecules whose lewis structure is similar to that of H2O.
The molecules having lewis structures similar to H2O are hydrogen sulfide and oxygen difluoride. They also have single bonds and lone pair electrons.
Conclusion:
If we will summarize the whole article then we will have the below highlighting and important points.
- H2O molecule comprises two hydrogen atoms and one oxygen atom.
- The bond angle among oxygen and hydrogen atoms in water (H2O) is 104.5°.
- The molecular structure of H2O is bent due to lone pair repulsion.
- There are a total of 8 valence electrons, out of which 4 electrons are used to form the O-H single covalent bonds.
We hope you are now clear about the lewis structure of H2O but if you have any doubts or questions then feel free to ask. We will solve them as soon as possible. Share this article with your friends and classmates.


